For those who want to download our presentation slide, please access the link below:
http://www.4shared.com/file/88573232/870fd36b/grp1_presentationmodified.html
Friday, February 20, 2009
Wednesday, February 18, 2009
GROUP 1
Chang Nyuk Shin (20775)
Fatin Nabila bt. Abdul Aziz (20959)
Julianne Anak Philip Attan (21169)
Mimi Zianna Panchar (21455)
Noor Farahana bt. Halim (21725)
Ng Siau Feun (16811)
Nurhafizah bt. Abdul Zamri (21988)
Peter Alphonso Anak Anthony Nyoel (22117)*
Siti Nurhanis bt. Basir (22381)
Tong King Hua (22562)
Fatin Nabila bt. Abdul Aziz (20959)
Julianne Anak Philip Attan (21169)
Mimi Zianna Panchar (21455)
Noor Farahana bt. Halim (21725)
Ng Siau Feun (16811)
Nurhafizah bt. Abdul Zamri (21988)
Peter Alphonso Anak Anthony Nyoel (22117)*
Siti Nurhanis bt. Basir (22381)
Tong King Hua (22562)
Coordination Bond and Coordination Compound
According to the Werner's theory of co-ordination compounds.
1) Metals possess two types of valencies:
a) Primary valency or ionizable valency. It is also referred to oxidation state.
b) Secondary valency which a metal atom or cation exercises towards neutral molecules or negative groups (ligands) in the formation of complex ions.The secondary valency is also called the coordination number.
Example: In [Pt(NH3)6]Cl4 secondary valency of Pt is 6
2) Primary valencies are satisfied by negative ions, secondary valencies may be satisfied by negative ions or neutral molecules.
3) Ligands satisfying secondary valencies are directed towards fixed positions in space giving a definite geometry to the complex, but the primary valencies are non-directional.
Six valencies are directed towards a regular octahedron while four are directed towards either a tetrahedral manner or square planar.
1) Metals possess two types of valencies:
a) Primary valency or ionizable valency. It is also referred to oxidation state.
b) Secondary valency which a metal atom or cation exercises towards neutral molecules or negative groups (ligands) in the formation of complex ions.The secondary valency is also called the coordination number.
Example: In [Pt(NH3)6]Cl4 secondary valency of Pt is 6
2) Primary valencies are satisfied by negative ions, secondary valencies may be satisfied by negative ions or neutral molecules.
3) Ligands satisfying secondary valencies are directed towards fixed positions in space giving a definite geometry to the complex, but the primary valencies are non-directional.
Six valencies are directed towards a regular octahedron while four are directed towards either a tetrahedral manner or square planar.
Before Werner, chemists defined the valence of an element as the number of its bonds without distinguishing different types of bond. However in complexes such as [Co(NH3)6]Cl3 for example, Werner considered that the Co-Cl bonds correspond to a "primary" valence of 3 at long distance, while the Co-NH3 bonds which correspond to a "secondary" or weaker valence of 6 at shorter distance. This secondary valence of 6 he referred to as the coordination number which he defined as the number of molecules (here of NH3) directly linked to the central metal atom. In other complexes he found coordination numbers of 4 or 8.
Today Werner's primary valence corresponds to the oxidation state, and the secondary valence is always called coordination number. The Co-Cl bonds (in the above example) are now classed as ionic, and each Co-N bond is a coordinate covalent bond between the Lewis acid Co3+ and the Lewis base NH3.
Coordination Compound
A coordination complex is the product of a Lewis acid-base reaction in which neutral molecules or anions (called ligands) bond to a central metal atom (or ion) by coordinate covalent bonds. Coordination compounds and complexes are distinct chemical species - their properties and behavior are different from the metal atom/ion and ligands from which they are composed.
A coordination compound is formed when groups of atoms, ions, or molecules chemically bond with each other by donating and accepting pairs of electrons. Groups donating electron pairs are called ligands. They are usually Lewis bases. Groups accepting electron pairs are often transition metal cations.
They are usually Lewis acids. Chemical bonds formed in this way are called coordinate-covalent, or dative bonds. As in any covalent bond, two electrons are shared between transition metal and ligand. But in a coordination compound, both electrons come from a pair found on the ligand .
The metal cation simply acts as the electron pair acceptor, itself donating no electrons to the bond. Because of the complicated nature of these arrangements, coordination compounds are often called coordination complexes or simply complexes. While nearly all cations can form coordination compounds with ligands. Ligands come in all shapes and sizes, though they are usually non-metals from the right side of the periodic table.
A coordination compound is formed when groups of atoms, ions, or molecules chemically bond with each other by donating and accepting pairs of electrons. Groups donating electron pairs are called ligands. They are usually Lewis bases. Groups accepting electron pairs are often transition metal cations.
They are usually Lewis acids. Chemical bonds formed in this way are called coordinate-covalent, or dative bonds. As in any covalent bond, two electrons are shared between transition metal and ligand. But in a coordination compound, both electrons come from a pair found on the ligand .
The metal cation simply acts as the electron pair acceptor, itself donating no electrons to the bond. Because of the complicated nature of these arrangements, coordination compounds are often called coordination complexes or simply complexes. While nearly all cations can form coordination compounds with ligands. Ligands come in all shapes and sizes, though they are usually non-metals from the right side of the periodic table.
Chelates
The term chelate was first applied in 1920 by Sir Gilbert T. Morgan and H. D. K. Drew, who stated: "The adjective chelate, derived from the great claw or chele (Greek) of the lobster or other crustaceans, is suggested for the caliperlike groups which function as two associating units and fasten to the central atom so as to produce heterocyclic rings."
Chelates play important roles in oxygen transport and in photosynthesis. Furthermore, many biological catalysts (enzymes) are chelates. In addition to their significance in living organisms, chelates are also economically important, both as products in themselves and as agents in the production of other chemicals.
A chelate is a chemical compound composed of a metal ion and a chelating agent. A chelating agent is a substance whose molecules can form several bonds to a single metal ion. In other words, a chelating agent is a multidentate ligand. The example of a simple chelating agent is ethylenediamine and porphyrin.
Chelating agents is used to detoxify poisonous metal agents such as mercury, arsenic, and lead by converting them to a chemically inert form that can be excreted without further interaction with the body, and has been approved by the U.S. Food and Drug Administration in 1991. Though they can be beneficial in cases of heavy metal poisoning, chelating agents can also be dangerous.
Chelating Agent: Ethylenediaminetetraacetic acid (EDTA)
EDTA is a widely used abbreviation for the chemical compound ethylenediaminetetraacetic acid. EDTA refers to the chelating agent with the formula (HO2CCH2)2NCH2CH2N(CH2CO2H)2. This polyamino carboxylic acid is widely used to sequester di- and trivalent metal ions (Ca2+ and Mg2+ for example). EDTA binds to metals via four carboxylate and two amine groups. EDTA forms especially strong complexes with Mn(II), Cu(II), Fe(III), Pb (II) and Co(III).
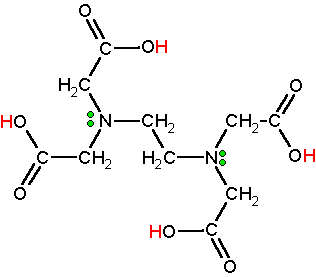
EDTA is used as a negative ion - EDTA4-. The diagram shows the structure of the ion with the important atoms and lone pairs picked out.
Function of EDTA
EDTA is a synthetic solution used in chelation therapy (pronounced key-lay-shun) for disorders including heart disease, circulatory problems, and lead/metal poisoning. Although EDTA chelation therapy has not been approved by the FDA for heart disease, it has been in the treatment of lead (and other metal) poisoning. In heart disease, chelation uses EDTA to bind with calcium (the glue that holds atherosclerotic plaque to artery walls), which breaks up plaque and carries the deposits out of the body. In many cases, EDTA chelation therapy is used as an alternative to heart bypass surgery.
This therapy is also used to promote healthy circulation, which may prevent gangrene and amputation. Since EDTA chelation therapy binds to and removes metals, it has been used to treat diseases such as Alzheimer's, cancer, macular degeneration (a progressive disease affecting vision), and lupus. It promotes a strong immune system, which aids in prevention and recovery from many illnesses.
Other uses of EDTA are industrial cleaning, as a detergent, in photography, pulp and paper industry, textile industry, agrochemical, hydroponics, also added as preservative for food, in cosmetic and many more.
Porphyrin
The Structure of porphyrin
The name porphyrin comes from a Greek word for “purple”. Porphyrin is a group of chemical compounds of which many occur in nature such as in green leaves and red blood cells. It contains four pyrrole rings. A pyrrole is a pentagon-shaped ring of four carbon atoms with a nitrogen atom at one corner (C4H5N). The porphyrins not only can be found all over the living world but they also bind to the metals. Metal ions such as magnesium (Mg), iron (Fe), zinc (Zn), nickel (Ni), cobalt (Co), copper (Cu), and silver (Ag) are grabed and holded by the four nitrogens in the middle of the porphyrin molecule (which act as teeth).
Importance of Porphyrin
When a porphyrin molecule grabs a metal, it acquires different properties and gets a different name. So, if the central metal is iron (Fe), the porphyrin complex is called ferroporphyrin, or heme. Hemoglobin has four of these heme groups. The iron ion of the heme group is responsible for binding the oxygen. The hemoglobin travels around the body, and when it reaches the tissues the iron in the porphyrin complex releases the oxygen to nourish them.
Moreover, chlorophyll, the all-important molecule that allows plants to do photosynthesis, and is responsible for plants' green color that is made up in part of a porphyrin molecule whose central metal ion is magnesium (Mg).The last but not the least, porphyrins which are universal, found in most living cells of animals and plants, where they perform a wide variety of functions.
Introduction to Vitamin B12
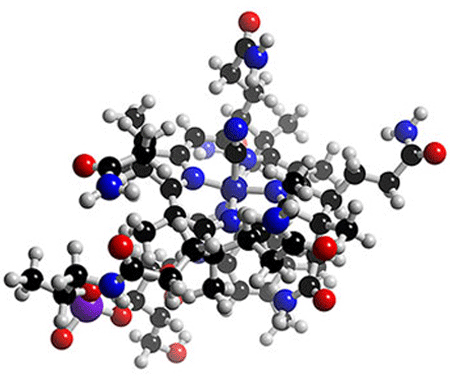
Molecular structure of vitamin B12.
B12 is a water soluble vitamin and is a member of the Vitamin B complex. Also known as Cobalamin (due to its cobalt factor), cyanocobalamin, hydroxocobalamin. Vitamin B12 is produced by the growth of certain micro-organisms and also occurs in the liver. Vitamin B12 has been prepared synthetically. Vitamin B12 cannot be made by plants or animals as only bacteria have the enzymes required for its synthesis. The total synthesis of B12 was reported by Robert Burns Woodward and Albert Eschenmoser, and remains one of the classic feats of organic synthesis.
Vitamin B12 is a vitamin with a key role in the normal functioning of the brain and nervous system, and for the formation of blood cell also growth and development in children. It is normally involved in the metabolism of every cell of the body, especially affecting DNA synthesis and regulation, but also fatty acid synthesis and energy production. Deficiency can cause anemia. Vitamin B12 neuropathy, involving the degeneration of nerve fibres and irreversible neurological damage, can also occur.
Typically, water-soluble vitamins cannot be stored by the body. Vitamin B12 is special, because the body can store it for years in the liver. Because of this, a vitamin B12 deficiency is very rare.
Sources of Vitamin B12
• Foods

Vitamin B12 is provided as a supplement in many processed foods, and is also available in vitamin pill form, including multi-vitamins. Vitamin B12 can be supplemented in healthy subjects also by liquid, strip, nasal spray, or injection and is available singly or in combination with other supplements. Cyanocobalamin is converted to its active forms, first hydroxocobalamin and then methylcobalamin and adenosylcobalamin in the liver. The sublingual route, in which B12 is presumably or supposedly absorbed more directly under the tongue, has not proven to be necessary or helpful. Injection is sometimes used in cases where digestive absorption is impaired, but there is some evidence that this course of action may not be necessary with modern high potency oral supplements (such as 500 to 1000 µg or more). Even pernicious anemia can be treated entirely by the oral route.These supplements carry such large doses of the vitamin that the many different components of the B12 absorption system are not required, and enough of the vitamin (only a few µg a day) is obtained simply by mass-action transport across the gut.
Vitamin B12 is naturally found in meat (especially liver and shellfish), milk and eggs. Animals, in turn, must obtain it directly or indirectly from bacteria, and these bacteria may inhabit a section of the gut which is posterior to the section where B12 is absorbed. Thus, herbivorous animals must either obtain B12 from bacteria in their rumens. Eggs are often mentioned as a good B12 source, but they also contain a factor that blocks absorption. Certain insects such as termites contain B12 produced by their gut bacteria, in a manner analogous to ruminant animals. While vegetarians usually get enough B12 through consuming dairy products, vitamin B12 may be found to be lacking in those practicing vegan diets who do not use multivitamin supplements or eat B12 fortified foods. Examples of fortified foods often consumed include fortified breakfast cereals, fortified soy-based products, and fortified energy bars. People on a vegan raw food diet are also susceptible to B12 deficiency if no supplementation is used.

Food Sources of Vitamin B12
Excellent Vitamin B12 Food Sources
• SupplementsVitamin B12 is provided as a supplement in many processed foods, and is also available in vitamin pill form, including multi-vitamins. Vitamin B12 can be supplemented in healthy subjects also by liquid, strip, nasal spray, or injection and is available singly or in combination with other supplements. Cyanocobalamin is converted to its active forms, first hydroxocobalamin and then methylcobalamin and adenosylcobalamin in the liver. The sublingual route, in which B12 is presumably or supposedly absorbed more directly under the tongue, has not proven to be necessary or helpful. Injection is sometimes used in cases where digestive absorption is impaired, but there is some evidence that this course of action may not be necessary with modern high potency oral supplements (such as 500 to 1000 µg or more). Even pernicious anemia can be treated entirely by the oral route.These supplements carry such large doses of the vitamin that the many different components of the B12 absorption system are not required, and enough of the vitamin (only a few µg a day) is obtained simply by mass-action transport across the gut.
Structure of Vitamin B12
Vitamin B12 is the member of Vitamin B complex. Vitamin B12 itself is commonly called cynocobalamin. Vitamin B12 is the most structurally complicated vitamin compound among the vitamins, made up of a class of chemically related compounds, and is directly involved in the metabolism of every single cell in the human body, even our DNA.
Vitamin B12 is the only known essential biomolecule with a stable metal-carbon bond, that is an organometallic compound and has corrin ring as a core. The central metal which is cobalt, can link to:
1. A methyl group- methylcobalamin
2. A 5’-deoxyadenosine at the 5’ position- adenosylcobalamin (coenzyme of B12)
3. A cyanide group- vitamin B12
Cyanocobalamin is one such compound that is a vitamin in this B complex, because it can be metabolized in the body to an active co-enzyme form. Since the cyanocobalamin form of B-12 is deeply red colored, easy to crystallize, and is not sensitive to air-oxidation, it is typically used as a form of B-12 for food additives and in many common multivitamins. However, this form is not perfectly synonymous with B-12, inasmuch as a number of substances (vitamers) have B-12 vitamin activity and can properly be labeled vitamin B-12, and cyanocobalamin is but one of them. (Thus, all cyanocobalamin is vitamin B-12, but not all vitamin B-12 is cyanocobalamin).
B-12 is the most chemically complex of all the vitamins. The structure of B-12 is based on a corrin ring, which is similar to the porphyrin ring found in heme, chlorophyll, and cytochrome. The central metal ion is cobalt. Four of the six coordination sites are provided by the corrin ring, and a fifth by a dimethylbenzimidazole group. The sixth coordination site, the center of reactivity, is variable, being a cyano group (-CN), a hydroxyl group (-OH), a methyl group (-CH3) or a 5'-deoxyadenosyl group (here the C5' atom of the deoxyribose forms the covalent bond with Co), respectively, to yield the four B-12 forms mentioned above. Historically, the covalent C-Co bond is one of first examples of carbon-metal bonds to be discovered in biology.
An important aspect of the corrin ring, when compared to the porphyrin, is the relative flexibility of the corrin system, the corrin ring is also less flat when viewed from the side than is a porphyrin ring. This adds up to some considerable differences between the chemistry of a cobalt porphyrin and a cobalt corrin. In addition, the corrin only has a conjugated chain around part of the ring system, whereas a porphyrin is delocalised around the whole four pyrolle rings.
Importance of Vitamin B12
To maintain a healthy body vitamin B12 is essential. Vitamin B12 plays role and helps to keep the red blood cells healthy and in so doing helps prevent heart disease. Vitamin B12 is needed for the process of converting carbohydrates, fats and proteins from food into energy. It also keeps the immune system healthy and functioning well.
The major important function of vitamin B12 is to form healthy red blood cells. However, all cells need vitamin B12 to keep them healthy. The white blood cells among others need vitamin B12 to ensure the immune system functions properly. The fatty layer is essential for all the nerves, but especially so for those in the brain. If there is not sufficient B12 in the body to create this protective layer then the brain will not function properly. Surprisingly the amount of vitamin B12 the body needs is relatively small,but needed on a regular basis. However, B12 on it's own is not enough because the body cannot absorb it easily. To help the body absorb B12 the stomach produces intrinsic factor, which enables more B12 to be absorbed. Pregnant women need more B12 for the baby to grow an develop properly because it is absorbing B12 during the pregnancy.
Overdose of Vitamin B12
There is a public opinion that the high dose of vitamin B12 as high doses of other vitamins can improve the activity of the immune system. The research doesn't have the certain results and the only conclusion. But it is necessary that high doses of the vitamins can lead to the side effects because of vitamin B12 toxicity.
An overdose of vitamin B12 is rare among the people. The symptoms of the overdose of vitamin B12 are numbness of any parts of body, itch, or a feeling of burning pain or pricking. The overdose of vitamin B12 may also lead to the following side effects: headache, giddiness, excitement, pains in the heart, the heart doesn't work normally.
The overdose of vitamin B12 cause allergy among the newborn babies and pregnant women. The overdose of vitamin B12 also can be noticed among the old people. It was discovered that about ten to thirty per cent people who over fifty years old are can suffer from the overdose of vitamin B12. It happens because of the fact that their organism can't absorb the vitamin B12. So, the vitamin B12 injections are recommended to them. The vitamin B12 is usually considered as non-toxic even when a healthy person takes it in the big quantity.
In the case of massive overdoses, toxic effects might occur due to the inactive ingredients in vitamin B12 supplements. Sometimes vitamin B12 comes in combination with other vitamins or other supplements. Of course, an overdose of such a product might cause overdose symptoms and effects due to the other components of the product.
An overdose of vitamin B12 is rare among the people. The symptoms of the overdose of vitamin B12 are numbness of any parts of body, itch, or a feeling of burning pain or pricking. The overdose of vitamin B12 may also lead to the following side effects: headache, giddiness, excitement, pains in the heart, the heart doesn't work normally.
The overdose of vitamin B12 cause allergy among the newborn babies and pregnant women. The overdose of vitamin B12 also can be noticed among the old people. It was discovered that about ten to thirty per cent people who over fifty years old are can suffer from the overdose of vitamin B12. It happens because of the fact that their organism can't absorb the vitamin B12. So, the vitamin B12 injections are recommended to them. The vitamin B12 is usually considered as non-toxic even when a healthy person takes it in the big quantity.
In the case of massive overdoses, toxic effects might occur due to the inactive ingredients in vitamin B12 supplements. Sometimes vitamin B12 comes in combination with other vitamins or other supplements. Of course, an overdose of such a product might cause overdose symptoms and effects due to the other components of the product.
The results of the overdose of vitamin B12 are its side effects. The vitamin B12 side effects are seldom noticed among the people with healthy kidneys. If there is something wrong with your kidneys and you take big doses of the vitamin B12 call the doctor if there are the vitamin B12 side effects, for example, numbness of any parts of body or itch. The overdose of vitamin B12 makes even the cancer appear. But it is one of the reasons in this case.
Itchiness
Deficiency of Vitamin B12
Clinical Manifestations of Vitamin B12 Deficiency
Hematologic
Megaloblastic Anemia
Megaloblastic Anemia Cells
Pellagrous
Allergies
Hematologic
- Megaloblastic anemia
- Pancytopenia (leukopenia, thrombocytopenia)
- Paresthesias
- Peripheral neuropathy
- Combined systems disease (demyelination of dorsal columns and corticospinal tract)
- Irritability, personality change
- Mild memory impairment, dementia
- Depression
- Cardiovascular
- Possible increased risk of myocardial infarction and stroke
Megaloblastic Anemia
Symptoms:
• Weak muscles
• Numbness or tingling in hands and feet.
• Difficulty in walking
• Nausea
• Decreased appetite
• Weight loss
• Irritability
Comparison between normal red blood cell and Megaloblastic Anemia blood cell
Megaloblastic Anemia Cells
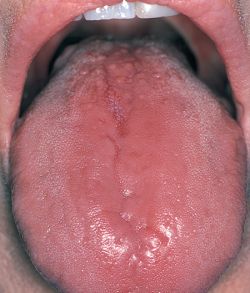
Pellagrous
Symptoms:
• Slick tongue
• Loss of appetite
Allergies
Vitamin B12 supplements in theory should be avoided in people sensitive or allergic to cobalamin, cobalt, or any other product ingredients. However, direct allergy to a vitaminor nutrient is extremely rare, and if reported, other causes should be sought.
Subscribe to:
Posts (Atom)