EDTA is a widely used abbreviation for the chemical compound ethylenediaminetetraacetic acid. EDTA refers to the chelating agent with the formula (HO2CCH2)2NCH2CH2N(CH2CO2H)2. This polyamino carboxylic acid is widely used to sequester di- and trivalent metal ions (Ca2+ and Mg2+ for example). EDTA binds to metals via four carboxylate and two amine groups. EDTA forms especially strong complexes with Mn(II), Cu(II), Fe(III), Pb (II) and Co(III).
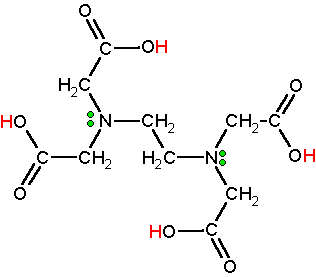
EDTA is used as a negative ion - EDTA4-. The diagram shows the structure of the ion with the important atoms and lone pairs picked out.
Function of EDTA
EDTA is a synthetic solution used in chelation therapy (pronounced key-lay-shun) for disorders including heart disease, circulatory problems, and lead/metal poisoning. Although EDTA chelation therapy has not been approved by the FDA for heart disease, it has been in the treatment of lead (and other metal) poisoning. In heart disease, chelation uses EDTA to bind with calcium (the glue that holds atherosclerotic plaque to artery walls), which breaks up plaque and carries the deposits out of the body. In many cases, EDTA chelation therapy is used as an alternative to heart bypass surgery.
This therapy is also used to promote healthy circulation, which may prevent gangrene and amputation. Since EDTA chelation therapy binds to and removes metals, it has been used to treat diseases such as Alzheimer's, cancer, macular degeneration (a progressive disease affecting vision), and lupus. It promotes a strong immune system, which aids in prevention and recovery from many illnesses.
Other uses of EDTA are industrial cleaning, as a detergent, in photography, pulp and paper industry, textile industry, agrochemical, hydroponics, also added as preservative for food, in cosmetic and many more.

No comments:
Post a Comment